Sterility Health Definition
The European Pharmacopoeia Ph. Sterile definition free from living germs or microorganisms.
In a man it is an inability to impregnate infertility physical condition physiological condition physiological state - the condition or state of the body or bodily functions.

Sterility health definition. Sterility can be defined as absence of viable Microorganism. However the conditions that guarantee absolute sterility are usually too harsh for active ingredients and the definition of sterility for a medicinal product must be defined in functional terms. Sterility - the state of being unable to produce offspring.
Sterility noun U UNABLE TO PRODUCE in animals and people the condition of being unable to produce young or in plants the condition of being unable to produce plants or crops the state of. Sterility definition is about the inability to conceive a child after having had intercourse for a year without any contraception. Health Canada is an active participating member of the Pharmaceutical Inspection Cooperation Scheme PICS.
Failing to produce or incapable of producing offspring a sterile hybrid compare infertile 2. This probability is commonly referred to as the sterility assurance level SAL of the product and is defined as the probability of a single viable microorganism occurring on a product after sterilization. Human sterility affects approximately 10 of couples of reproductive ages.
In a woman it is an inability to conceive. In the literature surgical asepsis and sterile technique are commonly used interchangeably but they mean different things Kennedy 2013. The classic clinical definition of infertility is the absence of conception after 12 months of regular unprotected intercourse.
The concept of what constitutes sterile is measured as a probability of sterility for each item to be sterilized. Key criteria for the appropriate selection and use of packaging materials will be reviewed followed by various professional guidelines for best practices related to packaging. About 30 of sterility cases are due to a problem of female origin an equal number is due to difficulties of male origin and finally 30 of cases are attributable either to the two members of.
The absence of viable contaminating microorganisms. What is Health Canadas position on pooling of samples within the same batch eg. Best Practice Guidelines for Cleaning Disinfection and Sterilization in Health Authorities - December 2011 Page 2 of 136 pages Foreword This document was originally developed in 2006 by the Ontario Provincial Infectious Diseases Advisory.
Eur does not mention explicitly a pooling of samples for testing for sterility. PRECAUTIONS AGAINST MICROBIAL CONTAMINATION. Medical Definition of sterile 1.
Seven samples in one pool for testing for sterility. 21 The sterility test applied to the fi nished product should only be regarded as the last in a series of control measures by which sterility is assured. The test for sterility is carried out under aseptic conditions.
Health Canada has adopted the PICS guidance Annex 1 Manufacture of Sterile Medicinal Products which describes how to manufacture sterile drugs in compliance with C02029 of. 22 Samples taken for ster ility test ing should be representat ive of the whole. Sterility noun U UNABLE TO PRODUCE in animals and people the condition of being unable to produce young or in plants the condition of being unable to produce plants or crops the state of.
It is acceptable to pool samples for sterility testing with the membrane filtration method. The test should be validated for the products concerned. Sterility assurance as it relates to the various sterilization processes and sterile packaging will be discussed.
Sterility can be defined as the freedom from the presence of viable microorganisms. Sterile technique is a set of specific practices and procedures performed to make equipment and areas free from all microorganisms and to maintain that sterility BC Centre for Disease Control 2010. In order to achieve such conditions the test environment has to be adapted to the way in.
It is well known however that many couples conceive without treatment after more than 12 months 1. Free from living organisms and especially microorganisms a sterile syringe a.

What Is Sterilization Definition Methods Types Video Lesson Transcript Study Com

What Causes Female Infertility Definition Signs Treatment

Medical Device Sterilization Ppt Video Online Download

Designating Of Medical Devices As Sterile And The Mdr
Https Www Mdpi Com 2673 4184 1 1 3 Pdf

Sterilization Validation Qualification Requirements Ppt Download

Sterility Or Infertility Are They The Same Or Is There Any Difference
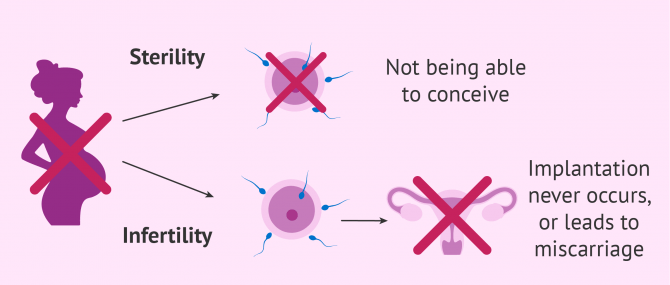
What S The Difference Between Infertility Sterility Subfertility

Pdf Defining Infertility A Systematic Review Of Prevalence Studies

Difference Between Sterile And Pyrogen Free
Https Www Mdpi Com 2673 4184 1 1 3 Pdf

Understanding The Difference Between Sterile And Clean

Sterility Maintenance Shelf Life Management

Cleaning Disinfection And Sterilization Validations Of Reusable Med

Definition Sterility It Is Means Women S Inability To Become Pregnant Due To Known Condition Such As Congenital Anomalies It Could Be Absolute Or Ppt Download
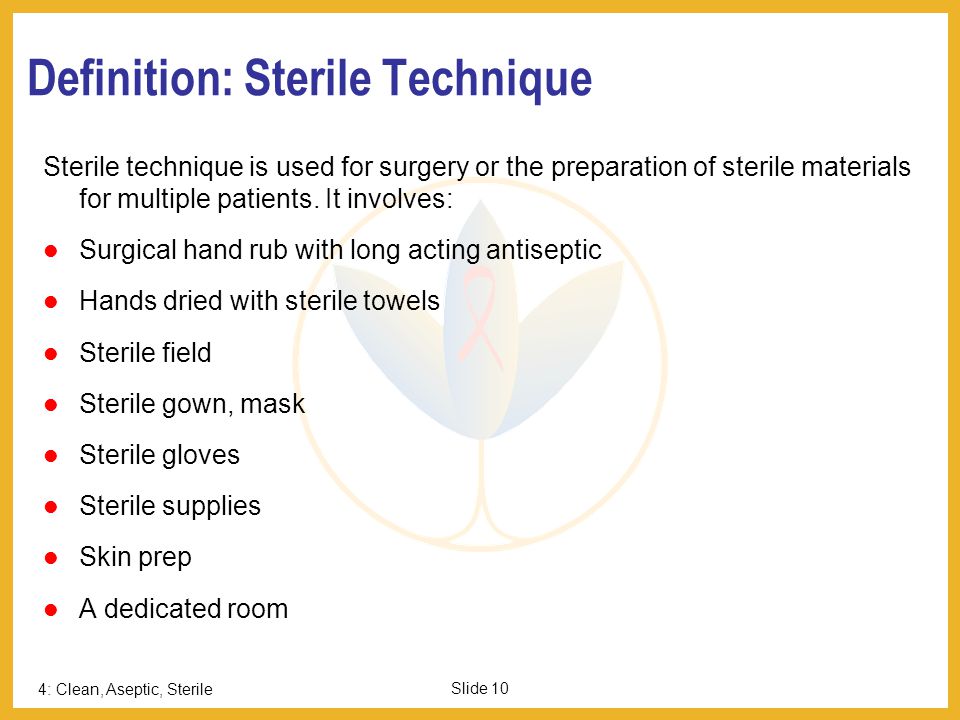
Clean Aseptic And Sterile Technique Ppt Download

Preventing Adverse Events During Sterilization And Disinfection Decisions In Dentistry
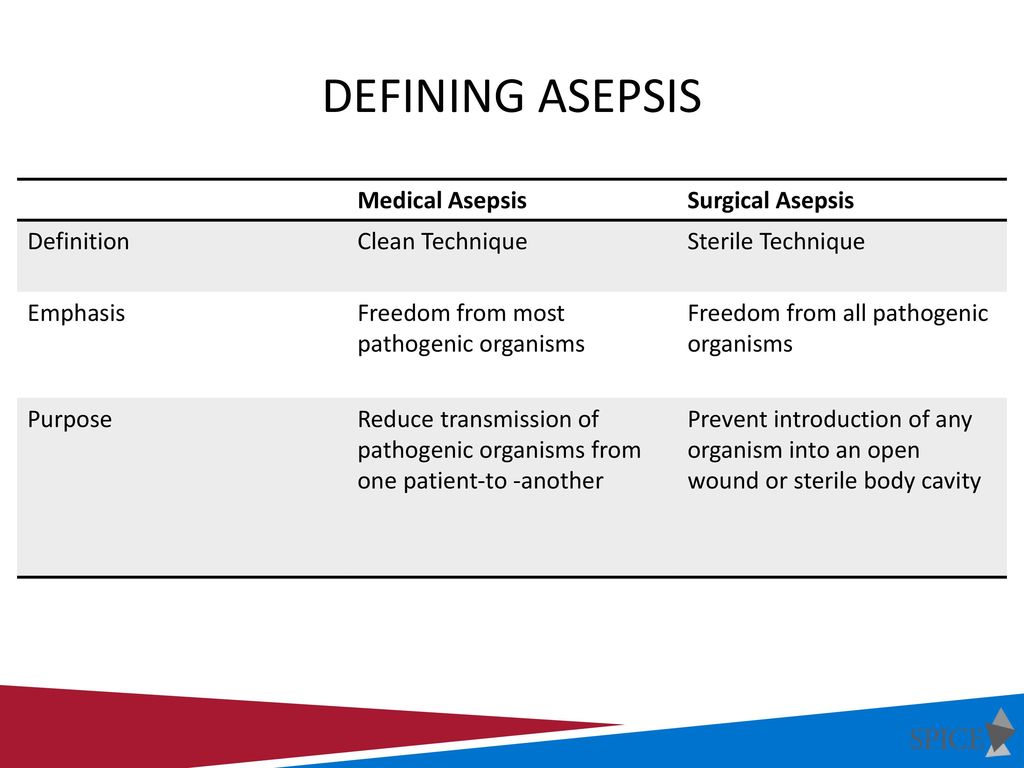
Principles And Practices Of Asepsis Ppt Download


Post a Comment for "Sterility Health Definition"